Polydienes
(Diene Elastomers, Synthetic Rubbers)
Properties
Diene elastomers are amorphous polymers having a glass transition temperature below room temperature, usually ranging between 170 and 250 K (-100°C and -25°C). They contain unsaturated sites and are often vulcanized (lightly cross-linked) to create an elastomeric network, which resists irreversible deformation below the break load.
The most important diene elastomers (synthetic rubbers) are styrene-butadiene rubber (SBR), polybutadiene (PBD), nitrile-butadiene rubber (NBR), polychloroprene (CR) and polyisoprene (IR). Among these rubbers, polybutadiene and butadiene copolymers (SBR) have the largest sales volume of the synthetic rubbers.
Styrene-butadiene rubber (SBR) is the largest-volume elastomer. It is a highly random copolymer of butadiene and 10 to 25 percent styrene. The addition of styrene improves the strength and abrasion resistance, reduces the price, and often improves the compatibiltiy with other materials in blends. SBRs have excellent resistance to brake fluids and good aging stability when protected by additives. Many grades of SBRs can withstand temperatures between 235 - 370 K for prolonged periods of time. However, their low temperature flexibility and tensile strength are less than that of natural rubber.
Nitrile-butadiene rubber (NBR) is another important copolymer. This rubber has good resistance to oil, fuel, and other chemicals, but is not resistant to weathering and ozone. The higher the nitrile content, the greater the resistance to oils but the lower the flexibility. In many cases, a medium nitrile content of 30 to 45 % is the best compromise between flexibility and chemical resistance. NBRs have better temperature resistance than PBD and can withstand temperatures of approx. 240 to 380 K.
Polybutadiene (PBD) is the second largest volume elastomer. It accounts for approximately 25 percent of the total global consumption of synthetic rubbers. The global polybutadiene production will soon reach more than 3.1 million tonnes (Source: PRWEB release from June 27, 2014). Due to its very low vinyl content it has a very low glass transition temperature (-90 °C), very high resilience and is probably the most elastic rubber. PBD also has good abrasion and tear resistance as well as low heat buildup, which is important for tire applications. Compared to SBR, it has a lower glass transition temperature, which gives it a lower rolling resistance. However, it has lower friction on wet surfaces than SBRs. For this reason, it is usually blended with SBRs and natural rubbers to achieve the best tire performance.
Polychloroprene (CR), also known as Neoprene, is the fourth largest volume diene elastomer. It exhibits good UV stability and has good chemical resistance to oxygen and ozone as well as oil, fuel, and many other chemicals. CR is known for its excellent abrasion resistance and toughness. Its mechanical properties are generally inferior to those of natural rubber but it has superior chemical resistance.
Polyisoprene (IR/NR) is another widely-used commercial rubber. It is either harvested in the form of latex from rubber trees (NR) or synthetically produced by polymerization of 1-methyl-1,3-butadiene (IR). Natural rubber is also known as India rubber or caoutchouc. Like most dienes, it has poor resistance to ozone, gasoline, and many organic solvents. It still finds many applications because of its high resilience, abrasion resistance, and strength. The largest portion of produced IR and NR is used for tires. It is usually blended with SBR and PBD rubber to achieve superior performance.
Butyl rubber (IIR), strictly speaking, is not a diene rubber, but rather a copolymer of isobutylene and small amounts of isoprene, which provide the unsaturated sites for vulcanization. It has unusually low gas permeability and outstanding resistance to UV, oxygen and ozone attack. Due to the low content of unsaturated bonds, it also has excellent chemical resistance and thermal stability.
A major drawback of synthetic and natural diene rubbers is the low heat resistance. Due to the double bonds in the backbone they are less thermally stable than saturated olefins. In fact, most diene rubbers are only long-term stable up to about 100°C.
COMMERCIAL POLDIENES
The most important commercial diene elastomer is SBR. In 2012, more than 5 million tons were produced worldwide (Source: Marktstudie Synthetische Elastomere, published by Ceresana, June 2013). Some major manufacturers and trade names are Goodyear ( Plioflex, Pliolite), Kraton Polymers (Kraton® D), and Firestone Polymers ( Stereon™, Duradene™). Many of these companies produce PBD rubbers (PR) as well. Important trade names are Diene™, and Budene.
Some major producers and brands of isoprene rubbers are Goodyear (Natsyn), and Kraton Polymers ( Cariflex™) .
Some important producers of chloroprene rubbers are DuPont (Neoprene), and Tosoh Corp. (Skyprene®).An important manufacturer of butyl rubbers (butyl, bromobutyl, chlorobutyl) is ExxonMoble (Exxon™ butyl rubber) .
Examples of Polydienes
| Polydiene | Cis Structure | Trans Structure | Trade Name |
| Polybutadiene (PBD) |
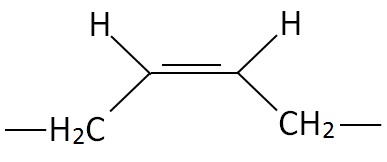
|
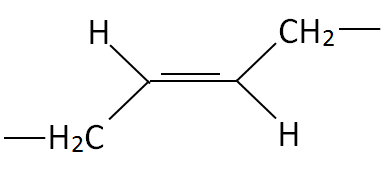
| Budene®, Taktene® |
| Polyisoprene (PI) |
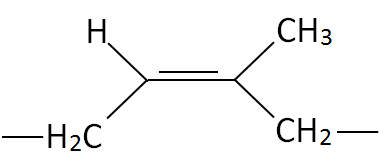
|
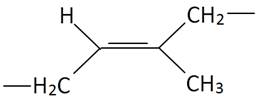
| SMR®, Natsyn® |
| Polychloroprene (CR) |
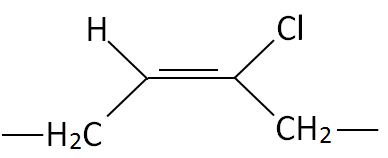
|
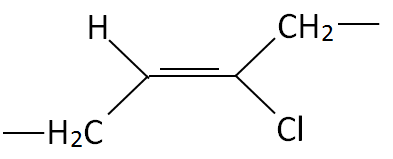
| Neoprene®, Bayprene® |
APPLICATIONS
The major use of SBR is in the production of tires (ca. 75 percent). Car tires often contain up to 50 percent SBR.
The styrene-butadiene ratio influences the properties of the tires: a higher styrene content improves the
hardeness but makes them less rubbery. Other important applications are shoe soles and heels, hoses, adhesives,
floor tiles, gaskets, and chewing gum.
SBR should not be confused with thermoplastic block-copolymers made from the same monomers,
the so called styrene-butadiene block copolymers (SBS copolymers).
The major use of butadiene rubber is in tires. Approx. 70 percent of the polymer produced goes into side walls and treads. PBD rubber is usually combined with other elastomers like natural rubber and SBR for tread applications. Other applications are golf ball cores, inner tubes of hoses for sandblasting, covers of hoses for pneumatic and water hoses, fuel for solid rocket boosters (in combination with oxidizers), and toughened plastics. About 25 percent of the total polybutadiene volume is used to improve the mechanical properties of plastics, in particular those of high-impact polystyrene (HIPS) and acrylonitrile butadiene styrene (ABS).
The majority of elastomers have either good chemical resistant to petroleum based lubricants or to oxygen/ozone; Polychloroprene is unusual as it offers good resistance to both. It therfore finds use in demanding applications such as automotive gaskets, o-rings, gasoline tubings, hoses, and corrosion-resistant coatings. Other important applications are diving suits, shoe heels, cable coatings, industrial hoses, gloves, adhesives and binders.
Another very resilient and chemical resistent rubber is nitrile-butadiene rubber (NBR). Because of its excellent oil resistance, it is used for gasoline hoses, O-rings, gaskets, V-belts and many other automotive rubber parts. It is also used for disposable laboratory gloves, synthetic leather, printer rollers, and as cable jacketing.
Butyl rubber is another elastomer that finds major use in the tire industry. Because of its low gas permeability, it is used for inner tubes of tires. It is also used for bladders in sporting balls, like footballs, basketballs, rugby, and soccer balls.